The pH scale: Basics simulation explores the pH of acids and bases in everyday life, and how pH is affected by dilution with water.
Click on the Link (opens in a new tab) pH Scale Basics
Topics
- pH
- Acids
- Bases
- Dilution
Description
Test the pH of everyday liquids such as coffee, spit, and soap to determine whether each is acidic, basic, or neutral. Investigate how adding more of a liquid or diluting with water affects pH.
Sample Learning Goals
- Determine if a solution is acidic, basic or neutral
- Place acids or bases in order of relative acidity or basicity
- Relate liquid color to pH
- Predict how solution volume or dilution with water will affect the pH of acids or bases
Insights into Use
After using indicators like litmus or pH paper, you may think that the colour of the liquid is related to pH. To tackle this idea, we show battery acid and drain cleaner with the exact same colour.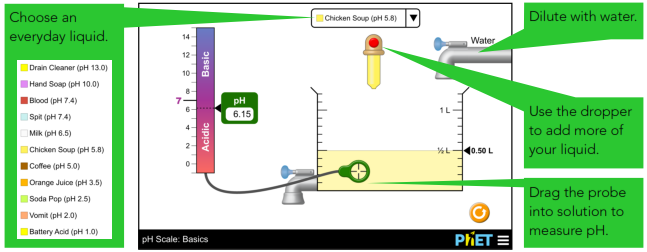
Model Simplifications
pH of everyday liquids: For liquids with a range of measured pH values, an average value from the literature was used. Dilution: The simulation does not account for different acid dissociation constants (Ka) for each liquid when calculating pH after dilution. We make the simplification that any increase in the concentration of the major ion is due to the ions already present in the added water. For example, if you add 100 mL of water to an acidic solution, then the number of moles of H3O+ an increases by 1 x 10 -8 mol. The concentration of the minor ion is then calculated using the self-ionization constant for water (Kw). These calculations account for the levelling effect of water.
Sample Challenge Prompts
- Classify solutions as acids or bases, given their pH.
- Predict if the pH of your solution will increase or decrease after you add water.
- Describe two different ways you could fill the beaker with a solution with pH 6.00. Is it possible to use hand soap to do this? Explain